For use of a Registered Medical Practitioner or a Hospital or a Laboratory only
Rx
Troxipide Tablets
TROXIP
COMPOSITION
Each film coated tablet contains
Troxipide…………….. 100 mg
Colour: Lake of Quinoline Yellow & Titanium Dioxide IP
DESCRIPTION
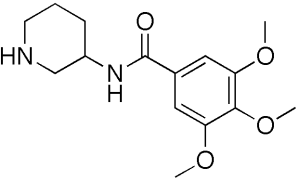
Troxipide, (±)-3,4,5-trimethoxy-N-3-piperidylbenzamide, is a gastroprotective agent with a Molecular formula of C15H22N2O4 and molecular weight of 294.3. The molecular structure of Troxipide is given below:
Fig: Troxipide
CLINICAL PHARMACOLOGY
Pharmacodynamics
Troxipide is known to treat gastritis and gastric ulcer, through a multimodal action. It is proposed to act by the inhibition of neutrophil functions in the gastric mucosa, thereby inhibiting Interleukin 8 (IL-8)-stimulated migration of neutrophils in the gastric mucosa, suppressing the formyl-methionyl-leucyl-phenylalanine (fMLP) or Platelet Activating Factor (PAF)-stimulated superoxide generation and decreasing the inflammation in mucosal tissues. Troxipide prevents mucosal fragility and disruption of gastric mucosal barrier by regeneration of collagen fibers and also increasing the gastric mucosal mucopolysaccharidosis content. Troxipide increases gastric mucosal blood flow, prostaglandin synthesis and glycoprotein excretion in the gastric mucosa.
Pharmacokinetics
Troxipide is well absorbed throughout the gastrointestinal tract after administration. Troxipide is detected in plasma from 0.05 hr after oral administration of 100 mg of film coated tablets, suggesting a rapid absorption. Bioavailability of Troxipide is 99.40%. A peak serum concentration of 1052.471 ± 51.9318 ng/ml is obtained within 3.042 ± 0.1896 hrs of drug administration and the resultant area under the curve is 8737.481 ± 315.4253 ng/ml*hr. It is found that, at any time, a mean concentration of 5.3 – 8.9 µg Troxipide is present per gram of tissue, which is capable of inhibiting the chemotactic migration and superoxide generation in the gastric mucosa. Thus, even 3 hrs after attaining peak serum levels, Troxipide is found in therapeutically active concentrations in the small intestine, liver and stomach. Troxipide has a half life of 7.615 ± 0.3782 hrs, and is mainly excreted in the urine (96%) as metabolites (61% after 24hrs and 87% after 48 hrs).
INDICATIONS
Troxipide is intended for use in the treatment of:
- Acute gastritis
- Acute exacerbation of chronic gastritis
- Peptic ulcers
DOSAGE AND ADMINISTRATION
The recommended dose of Troxipide is 100mg thrice daily for 8-12 weeks.
CONTRAINDICATION
Troxipide is contraindicated in patients showing hypersensitivity to Troxipide and in pregnant women.
PRECAUTIONS
Troxipide should be used with caution in children and pregnant women due to lack of safety data. It is known that sexual cycle dysfunctions occurred in rats treated with Troxipide. Hence, caution should be administered while treating women in the reproductive age group. Troxipide should be used with caution in breast feeding women; they should stop breast feeding when on the drug. Troxipide has to be cautiously used in geriatric population.
DRUG INTERACTIONS
There have been no reports of interactions with other drugs.
ADVERSE REACTIONS
Adverse reactions reported with Troxipide are generally mild to moderate in severity and disappear on discontinuation of the drug. The commonly reported adverse events include:
- Gastrointestinal effects like occasional constipation, abdominal bloating, and stomach discomfort
- Abnormality in liver functions (raised SGOT, SGPT, ALP levels)
- General malaise
STORAGE
Store in a cool and dry place
PRESENTATION
A blister strip of 10 tablets
MARKETED BY

(A JV Company of Emcure Pharmaceuticals Ltd.)
5119, ‘D’ Wing, Oberoi Garden Estate,
Chandivali, Mumbai 400 072, India


this is a best medicine in a case of gastric ulcer